There was a question on the forum recently about the differences between trace element calibration approaches. This Note aims to show that each approach is actually just a variation of the same approach with specific assumptions.
There are a couple of ways of calculating what are known in the laser ablation community as semi-quantitative concentrations (SQ concs). I’ll call the first approach the ``calibration curve’’ approach, and the second the Longerich approach, although the latter is actually just a specific case of the former, but let’s start with the calibration curve approach.
Note, I’m going to use a capital $C$ to denote SQ concentrations (i.e. a calculated concentration) as opposed to actual concentrations (i.e. an accepted value determined by another technique or by stoichiometry etc) which I’ll denote with a lower-case $c$.
Using a calibration curve, the SQ concentration can be calculated by:

where $C^i$ is the SQ concentration of element $i$, $R^i$ is the intensity (baseline-subtracted count rate) of element $i$, and, $a^i$ and $b^i$ are the slope and intercept of the calibration curve for element $i$.
For the case of multiple reference materials (RMs), a and b are usually calculated by some sort of least squares linear fit (or some variant of that). iolite provides four different fit types, but they are all linear and references are included in our 2023 paper.
Now to the Longerich SQ approach:

Here, the $R^{i}_{\mathrm{SAMP}}$ and $R^{i}_{RM}$ variables are still count rates, but for our sample and reference material, respectively, and $c^{i}_{RM}$ is the concentration of element $i$ in our reference material.
This equation isn’t directly in their 1996 paper, but it can be simplified out from the equations presented there, as I’ll hopefully show below.
If your experiment has just a single RM, and you assume that the intercept of the calibration curve is 0, Eqn 1 becomes:

The gradient ($a^i$) is defined as the rise over run, which for our calibration graph where the vertical axis is the intensity (in CPS) and the horizontal axis is the concentration, the gradient for a single RM calibration curve is simply rise over run, or intensity divided by concentration of our RM, i.e. $R^{i}_{RM}$ divided by $c^{i}_{RM}$, so our equation for a single RM becomes:

or, rearranging to get rid of the fraction on the bottom:

We’ll see this equation a few times below, so it’s probably worth taking an extra look at it. It basically says that, for example, if you get twice the count rate for your sample compared to your RM, you have twice the concentration of your RM for that element.
Hopefully with the above, you can see that the Longerich approach is the same as the calibration curve approach except that it assumes a zero intercept and uses the concentration and count rate of a single RM as the gradient.
iolite can use this form of the equation to calculate a concentration at any point in the experiment because we interpolate between RM measurements to get $R^{i}_{RM}$ at any point. This then accounts for the sensitivity drift that we commonly observe in our experiments, because we don’t assume a single gradient for our entire experiment, and by using splines you can choose how much influence individual RM measurements have on the overall spline by changing the spline type.
However, using the Longerich equation in this way only takes into account a single RM, so that’s why our paper took the calibration curve approach, and extended it into a third dimension to account for time effect such as sensitivity drift. The single RM approach described here is what the original “Trace Elements DRS” does.
Now let’s get back to the Longerich equation as presented in their 1996 paper, but using the same notation for count rates, sample and RMs as above. I’ll denote the concentration calculation using their approach, for element i, as $L^i$. The calculation of $L$ has a yield correction built into it to account for different ablation properties between the sample and the RM. Here it is in the original form:

where

If we combine these to make things clearer, we get
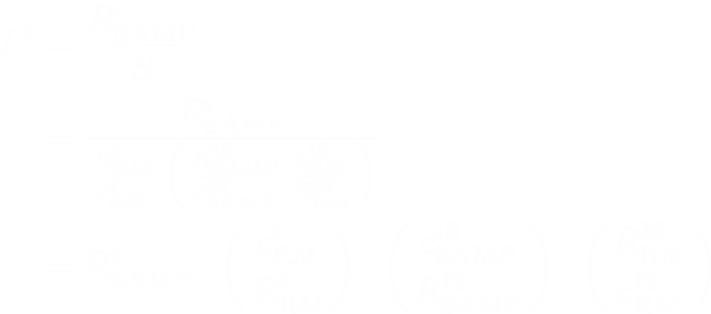
By regrouping these terms, we can hopefully make clearer the parts of the equation:

The first part is just the SQ concentration calculation, and the second part is a yield correction factor that we use to correct the SQ value.
If you look up the code for the 3D Trace Elements DRS, around Line 1382 in the current version (as of 2025-09-18) there is a “norm” factor. We’ll denote it as $n$ here.

If we substitute the SQ calc (Eqn 2 above) for $C^{IS}_{SAMP}$ (i.e. the SQ concentration of the internal std element), $n$ becomes
which you can see is just the internal standard correction factor in Eqn 8 above.
What Joe Petrus taught me was that you don’t have to use just a single internal standard element in these equations. In the sum normalisation approach, $n$ is actually

Multiplying SQ concentrations with this $n$ value produces the fully quantitative concentrations of the Longerich equation. That is,

If using all elements in the sample, the sum would be 100 wt%, which would be the c value in the equation above. This is then used in combination with the sum of all SQ concentrations to produce a fully quantitative concentration.
Using a single internal standard can use the same equation (it’s just the sum of a single element). This nicely combines the sum-normalisation approach and the Longerich approach, which are actually the same: the Longerich version is just a particular case where only one element is used for normalisation.
There are many advantages of using the sum normalisation approach, if you’re able to measure all element concentrations that can’t be assumed, but this usually requires a TOF instrument (or very long cycle times on a quad, which is usually limited to spot analyses). We discuss this in more detail in our webinar on trace element calculations.
Please note that there is a typo in our 2023 paper that also made it into our webinar presentation: the yield correction factor in the Longerich equation is flipped in our paper. The correct version is shown above.
Click here to discuss.